Abstract
There has been a progressive increase in therapies for patients with relapsed and refractory (R/R) peripheral T-cell lymphomas (PTCLs) in the last decade, including antibody-drug conjugates, epigenetic modifiers, small molecule inhibitors, and monoclonal antibodies (novel single agents, SA). Their comparative efficacy to conventional chemotherapy (CC) globally remains unknown. Here, we present preliminary results of an international collaborative study spanning multiple centers across 6 continents with diverse histological epidemiology, demographics, treatment patterns, and drug access.
We analyzed data from 324 patients with R/R PTCL enrolled between Jan 2010 and Dec 2020 [Brazil (n=120), Australia (AUS, n=69), COMPLETE registry USA (n=61), South Korea (SK, n=37), South Africa (SAF, n=24), and Saudi Arabia (SAU, n=13)], with ongoing participation from investigators in Italy, Chile, India, and Japan. Of 321 patients, 197 (61%) were refractory to 1st line therapy and 39% had relapsed. As expected, there was demographic heterogeneity globally. Median age at diagnosis was 54, with patients from AUS being significantly older (63). Majority were males except in SK (60% vs 25%). Overall, the most common histological subtypes were PTCL-NOS (40%), ALK- ALCL (16%), AITL (14%) and ENKTCL (12%). Exceptions included ENKTCL as the dominant subtype in SK (46%), ALCL in SAU (46%), and ATLL as the second most common subtype in Brazil (21%). Majority of patients had Ann Arbor stage III-IV disease (75%) and intermediate risk IPI (67%) and PIT scores (72%). SK and SAF had higher proportions of patients with early-stage disease and low risk IPI.
Overall, only 17% of patients underwent autoSCT consolidation in first remission with higher rates in SAU (50%) and SK (41%). Patients received either SA (25%) or CC (73%) for first retreatment. Brazilian patients received significantly less SA relative to CC (6% vs. 92%) in contrast with USA (49% vs. 51%), AUS (35% vs. 64%), SK (30% vs. 68%), SAF (29% vs. 71%) and SAU (15% vs. 85%). Globally, 38% and 22% achieved a second complete (CR) and partial remission respectively upon first retreatment. 25% of patients proceeded to alloSCT, with a significantly higher number in USA (71%) and SAF (43%). Second retreatment with SA vs. CC was received by 71% vs. 26% patients in AUS and 58% vs. 42% in USA as opposed to 35% vs. 65% in SK and 33% vs. 67% in SAF. Of interest, up to 48% of patients achieved a third CR to second retreatment. No patients in Brazil received second retreatment or further salvage therapy.
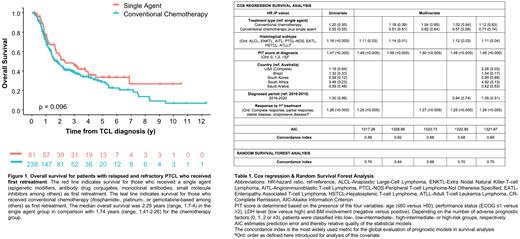
At median follow-up of 1.4 years (0.79-3.09), 32% of patients were alive, 61% had died, and 7% were lost to follow up. The median overall survival (OS) for patients with refractory disease (1.25 y) was inferior to those with relapsed disease (3.24 y; p<0.0001). When stratifying OS in each histological subtype by country, no statistically significant difference was found among patients with PTCL-NOS and ALCL entities. However, within the AITL subset, patients in Brazil exhibited a shorter survival compared to their counterparts in USA (p=0.045) and SK (p=0.045). In contrast, amid ENKTCL, patients in SK and Brazil had superior OS in comparison with USA (p=0.02 and 0.036) despite comparable age, stage, and IPI score. No statistically significant difference in OS was identified with respect to first retreatment with SA or CC overall and within individual histologies (Figure. 1). When stratified by calendar period of diagnosis (2010-2015 vs. 2016-2020), patients with ENKTCL (p=0.028) and ALCL (p=0.049) demonstrated an improved OS.
In separate models, overall risk of death was associated with histological subtype, PIT score, and lack of CR to 1st line treatment (Table 1). We then derived a final model by comparing AICs and concordance index across several models with combinations of these three measures. We verified the risk prediction of our final model with a second machine learning strategy using random survival forest.
In conclusion, this global study represents the first analysis of this type in R/R PTCL contrasting SA to CC and demonstrates that both strategies are comparable. Results confirm dismal prognosis for patients with primary refractory disease but also highlight improvements in OS in ALCL and ENKTCL in the last 5 years. We continue to enroll patients in our study and build better prediction models using state of the art synthetic intervention approaches to inform future clinical trials.
Disclosures
Chiattone:Takeda Oncology Brazil: Research Funding. Van Der Weyden:Cartherics Pty Ltd: Consultancy. Foss:Kyowa: Consultancy; Conjupro: Consultancy; Seagen: Consultancy, Speakers Bureau; Daiichi: Consultancy; Astex: Consultancy. Kim:Merck: Research Funding; Kyowa-kirin: Research Funding; Sanofi: Research Funding; Beigene: Research Funding; Boryong: Research Funding; Roche: Research Funding. Casadei:Incyte: Consultancy; BeiGene: Consultancy; Bristol Mayers Squibb: Consultancy; Takeda: Consultancy; Janssen: Consultancy; Gilead-Science: Consultancy; Abbvie: Consultancy. Zinzani:Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celltrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; University of Bologna: Current Employment. O'Connor:Kymera: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Stock; Dren: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Stock; Myeloid Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; Mundi Pharma: Consultancy; TG Therapeutics: Current Employment, Other: Stock. Marchi:University of virginia: Patents & Royalties: 3062/170 PROV; Celgene/BMS: Research Funding; Astex Pharmaceuticals: Research Funding; Merck: Research Funding; Myeloid Therapeutics: Ended employment in the past 24 months, Membership on an entity's Board of Directors or advisory committees; NomoCan Pharmaceuticals: Research Funding; Kyowa Kirin: Honoraria; Daiichi Sankyo: Other: Participation at advisory board. Jain:Crispr Therapeutics: Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acrotech LLC: Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Mersana Therapeutics: Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees, Research Funding; SIRPant Immunotherapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abcuro, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal